Welcome to UChicago PCCM Research!
Research defining the mechanisms, diagnosis, and treatment of lung disease and critical illness is a central mission of our group. This research is conducted in our clinical facilities as well as our basic research laboratories. Investigators are actively engaged in studying lung biology and cellular function related to critical illness, using techniques of cell and molecular biology, immunology, and genetics. Large and innovative clinical studies are also in place, with questions related to diagnosis and management of diverse diseases answered by accomplished clinical scientists observing individual patients and patient populations.
The strength of our research enterprise derives from the high degree of collaboration between basic and clinical scientists, and the joining of faculty and research associates with diverse training, expertise, and interests. The PhD, MD, and MD-PhD investigators in our group sustain this multi-faceted and dynamic approach to biomedical research. The essence of this activity is to ask important questions, and to answer them with whatever means are necessary—often employing the means of both clinical and basic science. The flow of ideas and findings is from bedside to bench and back, with each discovery adding to new questions requiring creative approaches to answer them. This exciting atmosphere provides abundant learning opportunities for students at every level of training, and we have successfully incorporated college, medical, and graduate students; post-doctoral scientists; residents; and fellows in our many programs.
News
Press release for Single cell metabolic imaging paper by PCCM Section!
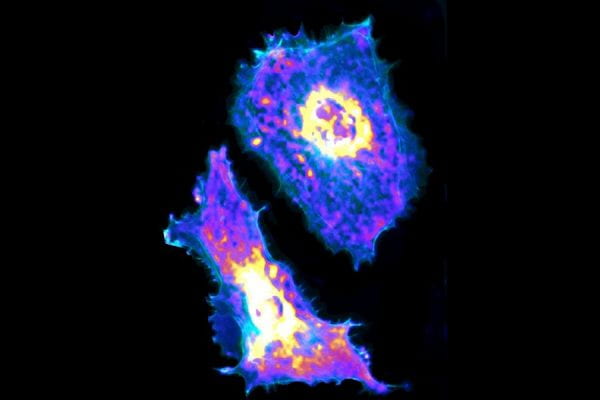
Single-cell metabolic imaging reveals a SLC2A3-dependent glycolytic burst in motile endothelial cells
New publication from the section!
Formally published! (and with an accompanying editorial: Subramanian, A., Becker, L.M. & Carmeliet, P. Endothelial metabolism going single. Nat Metab 3, 593–594 (2021). https://doi.org/10.1038/s42255-021-00399-3)
Citation:
Wu, D., Harrison, D.L., Szasz, T. et al. Single-cell metabolic imaging reveals a SLC2A3-dependent glycolytic burst in motile endothelial cells. Nat Metab 3, 714–727 (2021). https://doi.org/10.1038/s42255-021-00390-y
Abstract
Single-cell motility is spatially heterogeneous and driven by metabolic energy. Directly linking cell motility to cell metabolism is technically challenging but biologically important. Here, we use single-cell metabolic imaging to measure glycolysis in individual endothelial cells with genetically encoded biosensors capable of deciphering metabolic heterogeneity at subcellular resolution. We show that cellular glycolysis fuels endothelial activation, migration and contraction and that sites of high lactate production colocalize with active cytoskeletal remodelling within an endothelial cell. Mechanistically, RhoA induces endothelial glycolysis for the phosphorylation of cofilin and myosin light chain in order to reorganize the cytoskeleton and thus control cell motility; RhoA activation triggers a glycolytic burst through the translocation of the glucose transporter SLC2A3/GLUT3 to fuel the cellular contractile machinery, as demonstrated across multiple endothelial cell types. Our data indicate that Rho-GTPase signalling coordinates energy metabolism with cytoskeleton remodelling to regulate endothelial cell motility.